- 1. The Role of the Weighing Area in Pharmaceutical Manufacturing
- 2. Why Differential Pressure Control is Crucial in the Weighing Area
- 3. Principles of Differential Pressure Control in the Weighing Area
- 4. Essential Equipment for Differential Pressure Control
- 5. GMP-Compliant Layout Solutions for the Weighing Room
- 6. Common Mistakes in Differential Pressure Control in the Weighing Area
- 7. Frequently Asked Questions on Differential Pressure Control in the Weighing Area
- 8. Request a Consultation on Pressure Control for Pharmaceutical Weighing Areas
1. The Role of the Weighing Area in Pharmaceutical Manufacturing
The weighing area is one of the earliest and most critical links in the drug manufacturing process. This is where active ingredients and excipients are measured according to approved formulation standards. Any mistake at this stage can lead to serious consequences for the entire batch.
Why is the weighing area particularly important?
- Accurate determination of active ingredient quantities: The weighing step ensures each component is added according to the formula, directly impacting the drug’s efficacy and safety.
- Risk of dust dispersion and cross-contamination: Pharmaceutical ingredients are often in fine powder form, which can easily become airborne if not properly controlled. This can lead to cross-contamination between different batches — a severe violation of GMP standards.
- Frequently overlooked in GMP audits: Many facilities focus on production rooms and neglect the weighing area. However, GMP inspectors pay close attention to this zone due to its potential to affect the entire process.

The weighing area is not just about “measuring” raw materials — it marks the starting point of product quality and consistency. Proper investment in design, operation, and control — especially in terms of pressure and airflow — is critical to meet international standards for testing, production, and distribution.
2. Why Differential Pressure Control is Crucial in the Weighing Area
In cleanrooms for pharmaceutical production, airflow and differential pressure are vital for contamination control. In particular, the weighing area, where dust dispersion risk is high, must maintain stable pressure differences to meet GMP standards.
Maintain unidirectional airflow - prevent dust escape
Without proper pressure control, pharmaceutical dust can leak out and contaminate adjacent zones. By creating a pressure gradient, the HVAC system directs airflow from cleaner areas (higher pressure) to less clean areas (lower pressure). This helps:
- Pull fine dust particles into the filtration system instead of letting them escape into the environment.
- Keep contaminated air confined within the controlled space — especially important for high-toxicity or fine powder substances.
Protect personnel and production areas
The weighing room is typically adjacent to personnel airlocks, raw material storage, or pre-processing rooms. Without pressure balancing, dust can migrate into these spaces, putting operators at risk. Maintaining appropriate differential pressure:
- Creates a buffer to protect cleaner zones.
- Minimizes health risks for operators, especially during extended shifts.
Comply with GMP standards - avoid cross-contamination
Both EU GMP and WHO GMP require cross-contamination control, in which pressure control is a mandatory measure. Failure to maintain proper pressure differentials may cause:
- Non-compliance during GMP inspections.
- Production downtime to fix room design.
- Costly product recalls and damage to brand reputation.
3. Principles of Differential Pressure Control in the Weighing Area
To ensure product safety and a clean production environment, the weighing room must be designed with controlled pressure differentials between adjacent areas. The goal is to direct airflow in the proper direction — preventing dust from entering cleaner areas.
Apply slight negative pressure in the weighing room
In pharmaceutical plants, the weighing area is typically maintained at a lower pressure than adjacent spaces like airlocks, raw material storage, or main production rooms. This setup helps:
- Prevent dust-laden air from escaping the room.
- Contain particles within the space for filtration.

Note: For highly potent or easily dispersed ingredients, the weighing room may be designed as a dedicated negative-pressure zone, similar to an isolation room.
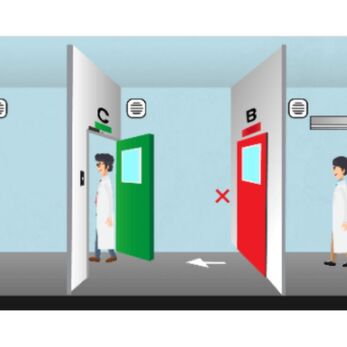
Airflow direction: from clean to less clean
According to GMP cleanroom design standards:
- Air should flow from cleaner areas to dirtier ones.
- This ensures that contaminants (dust, microbes, particulates) cannot flow upstream.
Example:
Production Room → Airlock → Weighing Room → Technical Corridor
Ideal pressure differential range: 10-15 Pa
International guidelines recommend that the pressure difference between two zones be kept in the range of:
- 10 - 15 Pascals (Pa) — sufficient to guide airflow without disrupting laminar flow.
If the pressure differential is too low: risk of backflow.
If too high: difficulty opening doors and operator discomfort.
Sample pressure levels in weighing zone layout:
|
Area |
Reference Pressure (Pa) |
|
Main Production Room |
+15 |
|
Personnel Airlock |
+10 |
|
Weighing Room |
0 or -5 |
|
Technical Corridor |
-10 |
From the table above, the weighing room is clearly positioned at the pressure low point — serving as a “buffer zone” to contain dispersed dust and protect adjacent critical areas.
4. Essential Equipment for Differential Pressure Control
To maintain stable pressure differentials in the weighing area, the cleanroom system must be equipped with synchronized measuring, control, and air supply equipment. Below are the essential components:
Dual-needle differential pressure gauge
This simple and easy-to-install device provides a visual display of the pressure between two adjacent rooms.
- The two needles allow operators to monitor the current pressure compared to the reference value (red needle).
- Ideal for on-site monitoring where no automated connection is required.
Digital pressure sensor
- Installed flush in the wall or ceiling, these sensors continuously record the pressure differential between spaces.
- They can be connected to a BMS/SCADA system to trigger automatic alerts when the pressure deviates from the safe range.
- High accuracy, suitable for modern pharmaceutical facilities.

Automatic pressure control system
- Combines sensors and a central pressure controller.
- When a deviation is detected, the system automatically adjusts the supply/exhaust airflow to restore balance.
- Commonly used in high-containment weighing rooms or ISO 7+ clean zones.
Airflow control valves (VAV/Dampers)
- Used to stabilize airflow rates in and out of each room.
- Can be manually operated or automated via signals from pressure sensors.
- Helps maintain unidirectional airflow and reduces backflow when doors are opened.
FFU or dedicated HVAC system for the weighing area
- FFU (Fan Filter Unit): Delivers top-down clean air while creating localized negative pressure.
- Independent HVAC system: Specifically designed for the weighing area to precisely control temperature, humidity, and pressure.
- Integrated with HEPA filters to remove active pharmaceutical dust at the source.
Choosing the right equipment and placing it correctly not only helps maintain stable pressure differentials but also ensures GMP compliance in terms of safety and cross-contamination control.
5. GMP-Compliant Layout Solutions for the Weighing Room
Designing a pharmaceutical weighing room requires more than just meeting space and functionality needs — it must strictly follow GMP principles for pressure control, airflow direction, and cleanability. Below are layout solutions that optimize operation and minimize cross-contamination risks:
Position the weighing room between two airlocks
- Personnel airlock and material airlock should be placed at both ends of the weighing room to manage entry/exit airflow.
- This layout helps create a stable pressure gradient, reducing the chance of reversed airflow when doors are opened.
- It also minimizes the entry of dust or microorganisms from external areas.
Ideal airflow direction:
Personnel Airlock (+10 Pa) → Weighing Room (0 or -5 Pa) → Material Airlock (+10 Pa)
Use buffer zones to prevent airflow crossover
- A buffer zone or sealed pass box should be installed between the weighing room and adjacent areas.
- Purpose:
- Reduce the time both doors are open simultaneously.
- Limit dust spread via airflow during door operation.
- The buffer zone can be equipped with interlocking doors to enhance control.
Construction materials: dust-resistant and easy to clean
- Walls: Should use flat, chemical-resistant panels with minimal gaps.
- Ceilings: Flush type, integrated with FFUs and cleanroom-grade LED lighting.
- Floors: Made of seamless, non-particulating materials such as antibacterial vinyl or epoxy coating.
All surfaces should be designed for easy cleaning and must not retain pharmaceutical dust — a key factor for meeting GMP cleanability standards.
Designing the weighing room based on the principle of “isolate - extract - block” not only ensures GMP audit readiness but also contributes to consistent product quality and operator safety.
6. Common Mistakes in Differential Pressure Control in the Weighing Area
Even with proper pressure control equipment installed, many pharmaceutical factories still experience airflow imbalances or fail GMP inspections. The root cause often lies in operational and design mistakes that are commonly overlooked.
Failure to perform regular pressure checks
This is a basic yet frequent issue.
When daily pressure monitoring is skipped or data logging is neglected, the following problems can occur:
- Pressure differentials go out of range during peak operations.
- Delays in detecting issues from exhaust fans, FFUs, or clogged filters.
Solution: Establish a routine pressure check for every shift, with a corresponding checklist for documentation.

Incorrect installation of gauges or sensors
If the high/low pressure sides are reversed or measurement points are poorly selected, the readings will not accurately reflect the actual airflow conditions.
This leads to incorrect system interpretations or improper alarm thresholds.
Note: Always consult HVAC drawings and airflow diagrams when installing pressure monitoring devices.
Lack of periodic equipment calibration
Differential pressure gauges and sensors are measurement instruments and must be calibrated regularly (typically every 6 or 12 months).
Allowing prolonged inaccuracy can result in false readings → pressure miscontrol.
Recommendation: Perform internal calibration or contract ISO 17025-certified labs for verification.
HVAC design lacks clear zoning for pressure control
A critical design flaw is failing to clearly separate supply and exhaust zones by pressure levels.
Consequences include:
- Difficulty adjusting pressure after commissioning.
- Failure to maintain unidirectional airflow (clean to dirty).
- Increased energy consumption due to compensating fan loads.
Solution: Design the HVAC system based on zoning principles, with dedicated pressure control valves for each area and proper airflow calculations from the start.
7. Frequently Asked Questions on Differential Pressure Control in the Weighing Area
How much pressure differential is ideal for the weighing room?
According to EU GMP and WHO GMP guidelines, the pressure difference between the weighing room and adjacent areas should be maintained at 10-15 Pa. This is sufficient to create unidirectional airflow and limit cross-contamination, while still allowing for easy door operation and HVAC system performance.
Is it mandatory to use a differential pressure gauge?
Yes. A differential pressure gauge (typically dual-needle) is required in most critical areas such as weighing rooms, airlocks, and compounding areas. This device provides instant visual monitoring and helps detect early deviations in airflow conditions.
Should the weighing room be under positive or negative pressure?
It depends on the nature of the material:
- For fine powders or high-toxicity substances, a slightly negative pressure environment is recommended to contain dust and prevent dispersion.
- For stable materials that are not easily airborne, neutral or slightly positive pressure may be used to protect the product from external contamination.
Note: Regardless of the pressure type selected, maintaining stable and continuous pressure control during operations is critical.
8. Request a Consultation on Pressure Control for Pharmaceutical Weighing Areas
Are you designing, renovating, or reassessing your pharmaceutical weighing room?
Don't let small pressure deviations cause GMP failures or serious cross-contamination risks.
VCR offers end-to-end solutions:
- Consultation on optimal airflow layout for weighing rooms
- Selection of appropriate pressure monitoring devices (dual-needle gauge, sensors)
- Dedicated HVAC and pressure control systems for weighing zones
- Operator training on pressure monitoring and regular calibration
Free on-site assessment and initial solution proposal within 24 hours.
Contact us now:
Hotline: 090.123.9008
Email: [email protected]
Website: https://phongsachduocpham.com/
Diep VCR
Vietnam Cleanroom (VCR) là một doanh nghiệp hàng đầu tại Việt Nam chuyên cung cấp thiết bị và giải pháp phòng sạch. Với hơn 10 năm kinh nghiệm phục vụ các dự án phòng sạch đạt tiêu chuẩn GMP, VCR tự hào mang đến các thiết bị kỹ thuật cao như: đồng hồ chênh áp, khóa liên động, đèn phòng sạch, Pass Box, FFU (Fan Filter Unit), buồng cân, HEPA Box, Air Shower, cửa thép phòng sạch, tủ cách ly (ISOLATOR), và nhiều loại phụ kiện chuyên dụng khác
Không chỉ là nhà cung cấp thiết bị, VCR còn là đơn vị phân phối độc quyền các sản phẩm từ các thương hiệu quốc tế như LENGE và BLOCK Technical, đồng thời cung cấp các giải pháp phòng sạch toàn diện cho các lĩnh vực như dược phẩm, điện tử, y tế, thực phẩm và mỹ phẩm. VCR có đội ngũ chuyên gia giàu kinh nghiệm, kiến thức chuyên sâu về phòng sạch, hỗ trợ tư vấn về tiêu chuẩn, thiết kế, thi công và vận hành phòng sạch theo chuẩn ISO, GMP, HACCP, ISO 14644
VCR hướng đến trở thành thương hiệu quốc dân trong ngành phòng sạch, với mạng lưới cung ứng rộng khắp, VCR có các văn phòng tại Hà Nội, TP. HCM, đáp ứng mọi yêu cầu từ xây dựng đến nâng cấp môi trường sản xuất đạt chuẩn
Email: [email protected]
Điện thoại: (+84) 901239008
Địa chỉ:
VP Hà Nội: 9/675 Lạc Long Quân, P. Xuân La, Q. Tây Hồ, TP. Hà Nội
VP Hồ Chí Minh: 15/42 Phan Huy Ích, P.15, Q. Tân Bình, TP.HCM
Hãy liên hệ với VCR để tìm hiểu thêm về lĩnh vực phòng sạch hiệu quả nhất nhé!