1. Why is a GMP audit failure dangerous?
A GMP audit is not just a procedure check; it is a test of the overall quality management capability of the factory. When an audit fails, the company faces several serious consequences:
- Damage to the company’s reputation in the eyes of regulators
A “non-compliant” rating reduces trust from regulators, partners, and customers. The brand may be labeled as unprofessional, making it harder to participate in tenders or sign contracts with major pharmaceutical companies. - Risk of production suspension or license withdrawal
In the case of serious violations, regulators have the right to suspend operations or even revoke the GMP certificate. This means products are no longer allowed to circulate, directly impacting the company’s revenue and cash flow. - Increased costs for remediation and re-assessment
After a failed audit, companies must reinvest in upgrading infrastructure, training staff, calibrating equipment, and standardizing procedures. Additional costs for reassessment and downtime in production can result in losses of millions.
In short, failing a GMP audit is not just about “losing points” with inspectors; it brings long-term financial, legal, and reputational risks for the factory.
2. The 5 most common GMP failures during audits
During audits, inspectors often focus on “critical points” directly related to product safety and GMP compliance. Below are five common issues that often result in a “non-compliant” conclusion:

1. Incomplete or inaccurate documentation
- Lack of standardized SOPs: Some companies still use internal procedures that are not approved or structured according to GMP.
- Failure to update to the latest versions: GMP EU/WHO guidelines are regularly updated, but internal documents may not be revised accordingly, leading to non-compliance.
2. Poor cross-contamination control
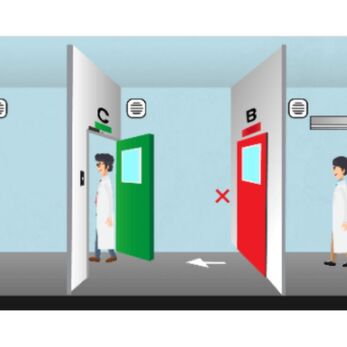
- Non-segregated cleanroom design: Weighing, compounding, and packaging areas are not clearly separated.
- Uncontrolled personnel and material flows: This creates a high risk of cross-contamination, especially in pharmaceutical and food factories.
3. Lack of timely calibration and maintenance of equipment
- Pressure gauges, Pass Boxes, and FFUs without regular maintenance logs → lack of transparency during inspection.
- No evidence of calibration under ISO/IEC 17025, making measurement data legally unreliable.
4. Inadequate sanitation and staff training
- Staff not following gowning procedures: incorrect order or misuse of cleanroom garments.
- Lack of GMP training records for operators, making it difficult to demonstrate competency during audits.
5. Non-compliance with environmental and HVAC requirements
- Unstable pressure differentials between rooms, leading to potential contamination.
- HEPA filters not tested regularly, reducing the ability to maintain GMP cleanliness levels.
Summary table of 5 common GMP failures
|
GMP Failure |
Consequence |
Corrective Action |
|
Inaccurate documentation |
Immediate audit failure |
Standardize & update SOPs |
|
Cross-contamination |
Risk of non-compliant product quality |
Redesign clean flows, segregate zones |
|
Lack of calibration |
Measurement errors |
Establish calibration & maintenance plan |
|
Insufficient staff training |
Incorrect production practices |
Conduct regular GMP training |
|
HVAC non-compliance |
Failure to maintain cleanliness levels |
Test and replace HEPA filters on time |
3. How to avoid failing a GMP audit
To ensure the factory is always prepared and capable of passing inspections, companies should implement preventive measures from the outset. Practical solutions include:

Build an internal GMP checklist before an official audit
- Create a checklist covering all aspects: SOP documentation, equipment calibration, cleanroom hygiene, and staff training.
- Conduct mock internal audits to identify weaknesses before regulators arrive.
Use certified cleanroom equipment
- Install FFUs (Fan Filter Units), Pass Boxes, Interlocks, and pressure gauges that are certified according to international standards.
- Prioritize equipment with monitoring logs and traceable data to demonstrate compliance.
Perform regular assessments with QA/QC and engineering teams
- QA/QC ensures adherence to SOPs and GMP standards.
- Engineering teams maintain equipment, HVAC systems, and HEPA filters in optimal condition.
- Collaboration between both teams helps detect and resolve issues promptly.
Maintain electronic records for easy traceability
- Digitize all documentation: SOPs, calibration certificates, maintenance logs, and equipment records.
- Use GMP-compliant document management software to save time, prevent data loss, and improve transparency during audits.
With thorough preparation in checklists, equipment, and documentation, companies not only reduce the risk of audit failure but also enhance professionalism and credibility in the eyes of partners and regulators.
4. FAQ - Frequently Asked Questions
What areas do GMP audits usually focus on?
→ Inspectors typically review key aspects such as SOP documentation, pressure differential control, cleanroom hygiene, staff training, and equipment calibration. These are direct indicators of GMP compliance.

How often should HEPA filters be tested in pharmaceutical plants?
→ Typically, every 6-12 months is appropriate, depending on the cleanliness level and GMP requirements. Some critical areas (such as weighing rooms and compounding zones) may require more frequent testing to ensure consistent air quality.
Is a Pass Box mandatory in GMP cleanrooms?
→ Yes. A Pass Box is essential for transferring materials between areas without disrupting airflow, thereby minimizing the risk of cross-contamination. In pharmaceutical plants, Pass Boxes are considered almost mandatory in GMP-compliant cleanroom designs.
5. Contact us for GMP-compliant cleanroom solutions
Thorough preparation for a GMP audit not only helps your factory pass inspections successfully, but also ensures a sustainable and reliable operation, minimizing production risks.
If you want to:
- Avoid the risk of audit failures
- Optimize your cleanroom design in compliance with EU/WHO GMP
- Equip your facility with certified cleanroom equipment: FFUs, Pass Boxes, Interlock systems, pressure gauges, HEPA filters...
Let VCR - Cleanroom Equipment be your trusted partner!
Contact us today for a free consultation:
Hotline: 090.123.9008
Email: [email protected]
Website: https://phongsachduocpham.com/
Diep VCR
Vietnam Cleanroom (VCR) là một doanh nghiệp hàng đầu tại Việt Nam chuyên cung cấp thiết bị và giải pháp phòng sạch. Với hơn 10 năm kinh nghiệm phục vụ các dự án phòng sạch đạt tiêu chuẩn GMP, VCR tự hào mang đến các thiết bị kỹ thuật cao như: đồng hồ chênh áp, khóa liên động, đèn phòng sạch, Pass Box, FFU (Fan Filter Unit), buồng cân, HEPA Box, Air Shower, cửa thép phòng sạch, tủ cách ly (ISOLATOR), và nhiều loại phụ kiện chuyên dụng khác
Không chỉ là nhà cung cấp thiết bị, VCR còn là đơn vị phân phối độc quyền các sản phẩm từ các thương hiệu quốc tế như LENGE và BLOCK Technical, đồng thời cung cấp các giải pháp phòng sạch toàn diện cho các lĩnh vực như dược phẩm, điện tử, y tế, thực phẩm và mỹ phẩm. VCR có đội ngũ chuyên gia giàu kinh nghiệm, kiến thức chuyên sâu về phòng sạch, hỗ trợ tư vấn về tiêu chuẩn, thiết kế, thi công và vận hành phòng sạch theo chuẩn ISO, GMP, HACCP, ISO 14644
VCR hướng đến trở thành thương hiệu quốc dân trong ngành phòng sạch, với mạng lưới cung ứng rộng khắp, VCR có các văn phòng tại Hà Nội, TP. HCM, đáp ứng mọi yêu cầu từ xây dựng đến nâng cấp môi trường sản xuất đạt chuẩn
Email: [email protected]
Điện thoại: (+84) 901239008
Địa chỉ:
VP Hà Nội: 9/675 Lạc Long Quân, P. Xuân La, Q. Tây Hồ, TP. Hà Nội
VP Hồ Chí Minh: 15/42 Phan Huy Ích, P.15, Q. Tân Bình, TP.HCM
Hãy liên hệ với VCR để tìm hiểu thêm về lĩnh vực phòng sạch hiệu quả nhất nhé!