- I. The Role of Air Filtration Systems in the Pharmaceutical Industry
- II. Technical Requirements for Air Filtration Systems in Pharma Facilities
- III. Standard Structure of Pharmaceutical Air Filtration Systems
- IV. Key Considerations When Standardizing Pharmaceutical Air Filtration
- V. Recommended Solutions - VCR's Air Filtration Equipment
- VI. Frequently Asked Questions About Pharmaceutical Air Filtration Systems
- VII. Need Help with a GMP-Compliant Air Filtration System?
I. The Role of Air Filtration Systems in the Pharmaceutical Industry
1. Why is air filtration a critical factor in pharmaceutical cleanrooms?
In pharmaceutical manufacturing, air is not only a medium but also a potential source of contamination. Ultrafine dust particles and airborne microorganisms can:
- Contaminate raw materials, intermediates, or finished products
- Alter the chemical properties of medicines, affecting therapeutic efficacy
- Lead to product recalls or pose health risks to consumers
Therefore, the air filtration system acts as the first line of defense, protecting product quality and ensuring production occurs in a strictly controlled environment.
2. Relationship between air filtration systems and GMP standards (WHO, EU, PIC/S)
All GMP standards emphasize environmental control, and air filtration is a mandatory component. Specifically:
|
Standard |
Air Filtration Requirements |
|
WHO-GMP |
Control of dust, microorganisms, and pressure differentials between production areas |
|
EU-GMP Annex 1 |
Defines cleanroom grades A/B/C/D according to ISO 14644; requires H13-H14 HEPA filters |
|
PIC/S |
Mandatory control of pressure differentials, airflow, and air change rates (ACH) |
Especially in critical areas like weighing rooms, packaging zones, and sterile production, a non-compliant air filtration system can cause the entire production process to fail GMP audits and severely impact inspection outcomes.

3. Risks if the air filtration system is non-compliant
Improperly designed or neglected air filtration systems can result in serious consequences:
- Cross-contamination between products due to ineffective pressure control
- Airborne microbial contamination exceeding allowed limits, especially in sterile areas
- GMP audit failure due to substandard ISO/Class ratings or lack of DQ/IQ/OQ/PQ documentation
- Loss of business credibility, affecting contract manufacturing or export agreements
II. Technical Requirements for Air Filtration Systems in Pharma Facilities
1. Cleanroom classification per ISO 14644 for different areas
ISO 14644-1 classifies cleanrooms based on airborne particle concentration, from ISO Class 1 to ISO Class 9. Common classifications in pharmaceutical environments include:
- ISO Class 5 (Grade A): Used in sterile zones, preparation of injectable products
- ISO Class 7-8 (Grade C/D): Used in packaging areas, weighing rooms, and corridors
Correct classification ensures the air filtration system is designed to meet standards efficiently.
2. Pressure differentials - Role and recommended values
Pressure differentials act as invisible barriers to control airflow direction. In pharmaceutical plants, maintaining appropriate pressure differentials helps to:
- Prevent dirty air from entering cleaner zones
- Avoid cross-contamination during production
Recommended pressure differentials:
- ≥10-15 Pa between clean and less clean areas
- ≥5 Pa between two clean areas with different contamination risks
Continuous monitoring and alert systems should be in place to ensure stability.
3. Air Changes per Hour (ACH) for each cleanroom grade
ACH is the number of times the entire air volume in a room is replaced per hour. It is essential to:
- Maintain stable dust and microbial concentrations
- Remove solvent vapors and airborne drug particles
Suggested ACH rates by cleanroom grade:
- Grade A / ISO 5: ≥240 ACH (FFU) or ≥90 ACH (HVAC)
- Grade C / ISO 7: 30-60 ACH
- Grade D / ISO 8: 10-20 ACH

4. Environmental parameters to be controlled
A compliant air filtration system must manage more than just particulate matter:
- Microbial control: Monitor airborne bacteria levels (CFU/m³)
- Humidity control: Maintain 45-65% RH to prevent condensation or dust release
- Temperature control: Typically kept at 20-24°C for product stability
- Integrated monitoring: Use sensors for pressure, temperature, and humidity, connected to BMS/SCADA systems
Summary Table: Environmental Parameters by Cleanroom Grade
|
Cleanroom Grade |
ISO Class |
Temperature (°C) |
Humidity (%) |
Pressure Differential (Pa) |
ACH (per hour) |
Microbial Count (CFU/m³) |
|
Grade A |
ISO 5 |
20-22 |
45-55 |
≥15 |
≥240 (FFU) |
<1 |
|
Grade B |
ISO 6 |
20-22 |
45-55 |
≥10 |
≥90 |
<10 |
|
Grade C |
ISO 7 |
20-24 |
45-60 |
≥10 |
30-60 |
<100 |
|
Grade D |
ISO 8 |
20-24 |
45-65 |
≥5 |
10-20 |
<200 |
Note: Values may vary based on the specific GMP guidelines (WHO, EU, PIC/S) and should be adjusted based on actual production needs.
III. Standard Structure of Pharmaceutical Air Filtration Systems
1. Overview of HVAC Air Filtration Systems in Pharmaceutical Facilities
Air filtration systems in the pharmaceutical industry are typically designed using an HVAC model with three filtration stages, integrated with airflow support devices and environmental monitoring systems.
Typical system layout:
- Fresh air → Pre-filter → Medium filter → AHU or FFU → HEPA filter → Cleanroom
- Return air → Filter → AHU → Recirculated back
- Exhaust air → Filter → Released to the environment (possibly through HEPA or UV filtration, depending on application)
Some specific areas may use standalone FFUs instead of central HVAC systems for added flexibility.
2. Position and Function of Key Components
Pre-filter:
- Location: At the intake of fresh or return air
- Function: Captures large particles, extends the lifespan of downstream filters
- Filter class: G4 - M5 (EN779)
Medium filter:
- Location: After the pre-filter, before AHU or FFU
- Function: Removes fine dust, powder, hair, fabric fibers
- Filter class: F7 - F9 (ISO ePM1 60-90%)
HEPA filter:
- Location: Installed at the final stage, before cleanroom air supply
- Function: Removes 99.97% of particles ≥0.3 micron
- Filter class: H13 - H14 (EN 1822)
- Can be mounted in HEPA boxes or inside FFUs
3. FFU vs. AHU - Active Clean Air Supply Options
FFU (Fan Filter Unit):
- Compact unit with built-in fan and HEPA filter
- Suitable for small rooms or areas requiring ISO 5-6
- Easy installation, energy efficient, simple maintenance
AHU (Air Handling Unit):
- Central air treatment station for entire facilities
- Controls temperature, humidity, and supports multi-stage filtration
- Ideal for large-scale operations requiring centralized control
4. Supply - Return - Exhaust Air System
- Supply air: Delivered to cleanroom through HEPA filtration
- Return air: Recaptured and reused after filtration
- Exhaust air: Discharged to the environment or isolation zone (if contamination is a risk)
Air return grills should be installed at low levels, and air supply diffusers at ceiling level to ensure unidirectional airflow and prevent cross-contamination.
5. Integration of Environmental Monitoring Systems
To comply with GMP, air filtration systems must integrate real-time monitoring tools:
|
Monitoring Parameter |
Corresponding Device |
|
Pressure differential |
Analog/digital manometers |
|
Temperature & humidity |
Sensors or BMS modules |
|
Airborne microorganisms |
Periodic microbial sampling devices |
|
Air velocity |
Anemometers or FFU-integrated sensors |
|
Alarm system |
Centralized SCADA or BMS alerts |
IV. Key Considerations When Standardizing Pharmaceutical Air Filtration
1. Select Air Filters Compliant with International Standards
Not all air filters are suitable for pharmaceutical use. Key requirements include:
- Compliance with EN 1822 or ISO 29463 for HEPA filters
- Certification for each filter unit: Efficiency scan, leak test
- Use of gaskets or gel seals to ensure air-tight installation
It’s recommended to use HEPA filters (H13-H14) in Grade A/B/C areas.
2. Establish a Maintenance and Calibration Schedule
Maintaining optimal system performance requires routine procedures:
- Cleaning or replacing filters based on service hours or pressure drop
- Regular HEPA filter efficiency testing (DOP/PAO) every 6-12 months
- Sensor calibration for temperature, humidity, and pressure once a year
Failure to maintain the system can compromise air quality and GMP compliance.

3. Proper Documentation per GMP Guidelines
Standardization goes beyond equipment; it includes robust documentation:
- URS (User Requirement Specification): Defines system expectations
- DQ/IQ/OQ/PQ: Phased validation during installation and operation
- SOPs for operation and maintenance: Must be well-trained and implemented
Complete documentation is essential to pass GMP inspections by any audit team.
4. Cross-contamination Control via Pressure and Airflow Design
Cross-contamination is a major risk in pharma manufacturing, especially for powder or injectable drugs.
To prevent it:
- Maintain proper pressure differentials between areas (≥10-15 Pa)
- Design unidirectional airflow, avoiding turbulence or unfiltered return air
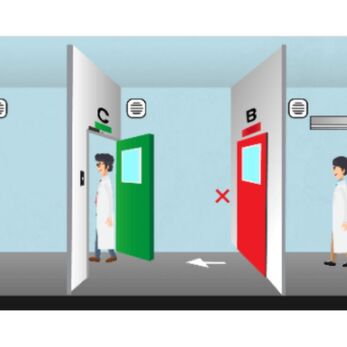
- Use interlocking doors, air showers, and buffer rooms to separate personnel and material flow
5. Integrate Air Filtration Design Early in Facility Planning
Many companies retrofit filtration systems after construction, leading to costly changes and compliance issues.
Suggested approach:
- Involve cleanroom design experts (e.g., VCR) from the planning phase
- Design air systems based on personnel flow, product flow, and process layout
- Optimize from the blueprint stage to ensure cost-efficiency and GMP readiness from day one
V. Recommended Solutions - VCR's Air Filtration Equipment
VCR is a leading provider of GMP- and ISO-compliant air filtration equipment for the pharmaceutical industry. Below are the recommended solutions for standardizing air filtration systems in pharmaceutical plants:
1. Specialized Air Filters: Pre-filter - Medium Filter - HEPA
Pre-filter (G4 - M5):
- Captures large dust particles, hair, insects, and fibers
- Extends the lifespan of downstream filters
Medium filter (F7 - F9):
- Removes fine dust, pharmaceutical powder
- Used in production areas and corridors
HEPA filter (H13-H14):
- ≥99.97% efficiency for particles ≥0.3μm
- Applied in Grade A/B/C cleanroom zones
- Certified and tested according to EN 1822
2. FFU - Standalone Filtration Solution for Individual Zones
- Fan Filter Unit with built-in fan and HEPA filter, ceiling-mounted
- Suitable for weighing rooms, injection preparation areas, and small production zones
- Easy to upgrade, does not rely on central HVAC
- Supports cleanroom control up to ISO 5 / Grade A

3. HEPA Box - Flexible Filtration Option
- Ceiling-mounted or suspended HEPA filter box
- Ideal for Grade C/D areas where cost-efficiency is important
- Can be integrated with pressure gauges and particle sensors
4. Differential Pressure Gauges - Continuous Monitoring
- Measures and displays pressure differentials between clean and non-clean areas
- Available in mechanical, digital, or RS485 sensor versions for BMS integration
- Supports alarm systems for abnormal pressure readings
5. Interlocking Doors and Air Showers - Personnel Flow Control
Cleanroom interlocks:
- Prevent simultaneous opening of multiple doors, ensuring room pressure integrity
- Can include RFID access control and alarm functions
Air showers:
- Blows off electrostatic dust before entering clean zones
- Reduces the risk of introducing microorganisms or contaminants from outside
VI. Frequently Asked Questions About Pharmaceutical Air Filtration Systems
1. What filtration levels are required for a GMP pharmaceutical facility?
→ Depending on the area, facilities typically use pre-filters (G4), medium filters (F7-F9), and HEPA filters (H13-H14). These ensure cleanroom levels of Grade A/B/C in line with EU-GMP standards.
2. Should I choose FFU or a centralized HVAC system?
→ FFUs are suitable for specific areas like weighing rooms due to ease of installation and independent control. Central HVAC is better for large-scale facilities needing synchronized control.
3. What validations are required for air filtration systems to meet GMP?
→ Full validation includes DQ (Design Qualification), IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification). HEPA filters must be DOP/PAO tested, and all key parameters (pressure, temperature, humidity) should be continuously monitored.
4. How can I verify that the air filtration system is performing effectively?
→ Performance can be confirmed through stable pressure differentials, cleanroom class compliance (ISO), correct air change rates, and passing results in microbial testing and HEPA filter validation.
VII. Need Help with a GMP-Compliant Air Filtration System?
If you're building or upgrading a pharmaceutical plant, don't let the air filtration system become a weakness during GMP audits. VCR offers end-to-end solutions:
- Certified air filtration equipment (pre-, medium-, and HEPA filters)
- FFUs, HEPA Boxes, pressure gauges, interlocking doors, and air showers
- Consulting, design, and full IQ - OQ - PQ validation services
VCR - A trusted cleanroom solution provider for the pharmaceutical industry in Vietnam.
Hotline: 090.123.9008
Email: [email protected]
Website: https://phongsachduocpham.com/
Diep VCR
Vietnam Cleanroom (VCR) là một doanh nghiệp hàng đầu tại Việt Nam chuyên cung cấp thiết bị và giải pháp phòng sạch. Với hơn 10 năm kinh nghiệm phục vụ các dự án phòng sạch đạt tiêu chuẩn GMP, VCR tự hào mang đến các thiết bị kỹ thuật cao như: đồng hồ chênh áp, khóa liên động, đèn phòng sạch, Pass Box, FFU (Fan Filter Unit), buồng cân, HEPA Box, Air Shower, cửa thép phòng sạch, tủ cách ly (ISOLATOR), và nhiều loại phụ kiện chuyên dụng khác
Không chỉ là nhà cung cấp thiết bị, VCR còn là đơn vị phân phối độc quyền các sản phẩm từ các thương hiệu quốc tế như LENGE và BLOCK Technical, đồng thời cung cấp các giải pháp phòng sạch toàn diện cho các lĩnh vực như dược phẩm, điện tử, y tế, thực phẩm và mỹ phẩm. VCR có đội ngũ chuyên gia giàu kinh nghiệm, kiến thức chuyên sâu về phòng sạch, hỗ trợ tư vấn về tiêu chuẩn, thiết kế, thi công và vận hành phòng sạch theo chuẩn ISO, GMP, HACCP, ISO 14644
VCR hướng đến trở thành thương hiệu quốc dân trong ngành phòng sạch, với mạng lưới cung ứng rộng khắp, VCR có các văn phòng tại Hà Nội, TP. HCM, đáp ứng mọi yêu cầu từ xây dựng đến nâng cấp môi trường sản xuất đạt chuẩn
Email: [email protected]
Điện thoại: (+84) 901239008
Địa chỉ:
VP Hà Nội: 9/675 Lạc Long Quân, P. Xuân La, Q. Tây Hồ, TP. Hà Nội
VP Hồ Chí Minh: 15/42 Phan Huy Ích, P.15, Q. Tân Bình, TP.HCM
Hãy liên hệ với VCR để tìm hiểu thêm về lĩnh vực phòng sạch hiệu quả nhất nhé!